Marrow CellutionTM bone marrow aspiration device is a minimally invasive procedure that uses patented technology to harvest high quality stem and progenitor cells from various geographies within the marrow space while limiting peripheral blood contamination. [More information]

The Marrow CellutionTM – bone marrow Aspiration System is intended for use for autologous bone marrow aspiration and maximizes stem cell and progenitor cells collected. It allows the user to aspirate in a measured and controlled manner over a large geography within the marrow space. Marrow CellutionTM is available in 11 Gauge and 13 Gauge diameter and includes an introducer needle, sharp and blunt stylet, aspiration cannula and 10 ml syringe. Marrow CellutionTM also comes in multiple lengths and is designed for use in the Illiac Crest, Pedicle, Calcaneous or Tibia.
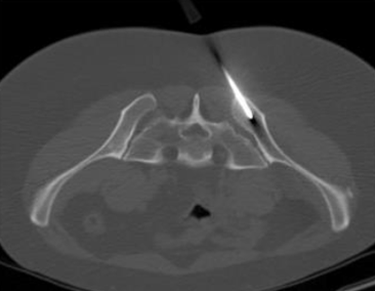
Traditional bone marrow aspiration needles aspirate primarily through an open-ended cannula. Because fluid under force follows the path of least resistance the traditional trocar aspiration leads to excess peripheral blood contamination and inadequate collection of key stem and progenitor cells as well as an overall diminished cellular yield. For this reason, a high volume of bone marrow aspirate must be collected from multiple locations and then manipulated (i.e., centrifuged or chemical separation in a lab) before being applied for regenerative therapies.

The innovative design of the Marrow CellutionTM Aspiration System offers two key, unique design features that maximizes cellular yield, minimizes patient discomfort, and reduces the length of time necessary to achieve greater results while maintaining the sterile field.
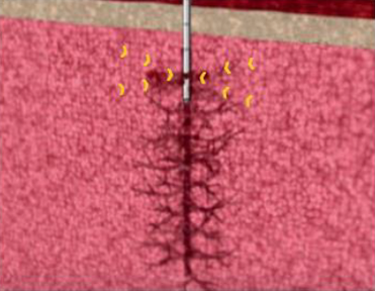
Marrow stem cells heal tissue as an integral part of a patient’s immune system and only marrow stem cells are powerful enough to reconstitute an entire immune system in an oncology setting. Through cytokine release and cell to cell contact, bone marrow stem cells orchestrate the transition from inflammation to proliferation and remodeling in the healing cascade. Mechanically sourcing and placing the cells responsible for this transition is exactly mimicking and supplementing your body’s natural reparative process.
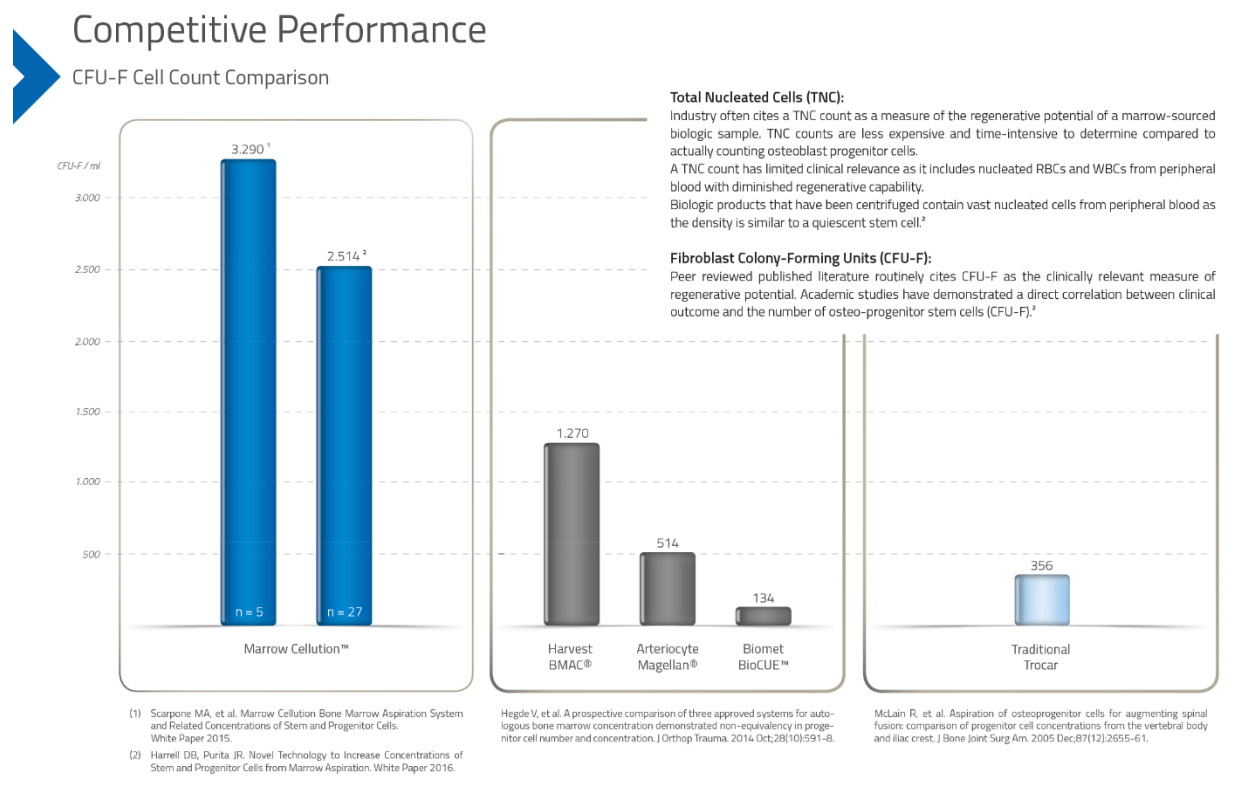
The Marrow CellutionTM System delivers a better regenerative solution with more stem cells at a reduced cost compared to industries leading solutions
Centrifugation systems typically discard 80% of the aspirate due to the high levels of peripheral blood contamination. Worse, approximately 40% of the desired cells are discarded because their density is similar to the undesired red cell centrifuge component and thus discarded, substantially limiting the regenerative potential of the sample.
Centrifugation systems require passing the BMA off the sterile field for processing and back on for implantation. The Marrow CellutionTM System eliminates the additional steps where infection concerns need to be managed.
Centrifugation systems require at least 10% dilution by volume of anti-coagulant to allow the sample to separate as well as another 10% dilution in the form of a neutralizing agent such as thrombin and calcium chloride in order for the marrow to clot in the graft. The Marrow CellutionTM System eliminates these requirements.
Traditional trocars require several penetrations and additional preparation while also requiring a traditional centrifugation which typically requires more than 20 minutes of spin time not to mention the additional personnel (perfusion) and support time needed for preparation and cleanup of the equipment. Marrow CellutionTM can obtain higher CFU-f counts with one penetration and 0 spin time.
Traditional protocols require the marrow to be filtered prior to centrifugation. Marrow CellutionTM achieves a bone marrow aspirate that allows cells bound within a cell aggregate to be delivered to the patient when mixed with graft material or injected directly. This is not the case when aggregates are filtered out prior to centrifugation. Filtering takes additional time but more importantly filtering reduces regenerative potential.
Dr. Dan Kuebler Professor of Biology, Chair of the Biology Department; Franciscan University
A larger-volume of aspirate (more than 2mL) from a given site is contraindicated with the additional volume contributing little to the overall number of bone-marrow cells and results principally in unnecessary blood loss.
MUSCHLER G, et al Aspiration to Obtain Osteoblast Progenitor Cells from Human Bone Marrow: The Influence of “Aspiration Volume The Journal of Bone and Joint Surgery;” VOL. 79-A, NO. 11 Cleveland Clinic